What is Parallel Imported Medicine?
Medication that is dispensed from one of our pharmacy partners in the United Kingdom may be a parallel imported product. Parallel imported medicine is 100% identical to the original medicine - even if the product is sold under a different name, comes in packaging that is different from the UK brand version, has non-English language packaging, or has been relabeled. All parallel imported medicine in the UK pass through the same rigorous Medicines and Healthcare products Regulatory Agency (MHRA) quality assurance measures that protects medication that is manufactured inside the UK.
Parallel imported medicine is not a generic product, because the drug company that holds the patent has manufactured it or has licensed another company to manufacture. The only difference may be the packaging because some products have different names in different countries, or a UK label may have been placed over the European version. Parallel imported product is standard practice in the UK and commonly purchased by their citizens at local pharmacies.
Here are some visual examples of parallel imported medication.
| UK Brand Medicine | Parallel Imported Medicine |
|---|---|

|

|

|
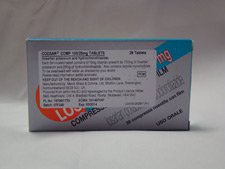
|
Parallel importing happens because the United Kingdom is a member of the European Union (EU) and European Union countries are authorized to import medication from other member states for sale in their jurisdictions, repackage the medication and offer it for sale to their citizens. Some drug companies make the same drug at factories in the UK and abroad. For example, a drug like Singulair is made in a number of countries as well as in the UK. Parallel importing is when a drug wholesaler buys from a drug company’s European factories to supply to UK pharmacies. So, Singulair or other parallel imported medicine may have been made in a number of countries, including the UK, Netherlands and Italy, but is the exact same product regardless of packaging.



